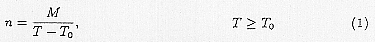
AIM Project Team is composed with Tsuneyuki Morita, Mikiko Kainuma, Hideo Harasawa and Keiko Kai from National Institute for Environmental Studies and Yuzuru Matsuoka from Faculty of Engineering, Kyoto University. This paper is one of interim reports on work of the AIM Project.
Global Warming Response Team, Global Environment Group, National Institute for Environmental Studies, 16-2 Onogawa, Tsukuba 305, Japan
Telephone: +81 298516111 Telefax: +81 298582645
Department of Environmental and Sanitary Engineering, Faculty of Engineering, Kyoto University, Sakyoku, Kyoto 606-01, Japan
Telephone: +81 757535167 Telefax: +81 757535175
In this study, we focus on one of the socio-economic impacts of global warming, that of human health and quantitatively estimate the increased risk of malaria infection. The climate change expected in the next century will cause a surface temperature rise, an increase in precipitation and a sea level rise. These are expected to affect human lives in various ways such as land loss, impacts on agricultural productivity, natural ecosystem and human health. In this study, we will first describe the estimated magnitude of these impacts and then focus on the health risk by malaria. For malaria, in particular, although the impacts on propagation of Anopheles (the disease vector) and so on are considered to greatly increase the likelihood of the disease, these factors have never been evaluated quantitatively. In this study, we estimate the increase in risks to health caused by malaria. First, we estimated the current and post-global warming distribution of Anopheles by calculating its eco-climatic matching using annual and daily temperature and soil moisture as basic parameters. These parameters were deduced from the water balance model with General Circulation Models' results. Then we quantitatively analyzed the change in malaria endemicity taking into account the relationship between temperature and the duration of sporogony of malaria parasites in the Anopheles. We concluded that climate change caused by a doubling of CO2 will allow the malarial area to increase by 10%~30% (population percentage).
By March 1994, more than 50 nations had ratified the Framework Convention on Climate Change which was adopted at the Earth Summit in 1999 and agreed to apply its provision. The objective of this convention is to achieve stabilization of greenhouse gas concentrations in the atmosphere at a level that would prevent dangerous anthropogenic interference with the climate system.
Researchers around the world were requested to quickly identify the routes of, and to evaluate the degree of, dangerous interference which would be caused by climate change (Bolin, 1994). Determining the allowable range of climate change is one of the ultimate aims of the research on global warming and can not be completed lustily. The range of impacts is quite diverse so it is not possible to treat them all in the same way. Many problems remain unsolved if we try to estimate them quantitatively. However, there is some research which has tried to comprehensively quantify global warming damage. Nordhaus (1991), Cline (1992), Fankhauser (1992), Titus (1992) and Hohmeyer et a!. (1992) estimated the accumulated damage caused by a doubling of CO2. Nordhaus, Cline and Titus estimated the situation for the U.S., while Fankhauser and Hohmeyer et al. looked at the global situation. Wide range of impacts, e.g. land loss caused by sea level rise, agriculture, forestry and fisheries, natural ecosystem, water use, human health, air and water pollution and intensification of natural disasters were considered and the economic damages of these impacts summed up to 1.5% of the GNP, under the assumption of temperature rise to be 2.5°C (a global average) and the sea level rise to be 50 cm. Nordhaus, Cline and Titus estimated almost the same level of damage (1.0%~2.5% of GNP for the U.S.). On the other hand, Hohmeyer et al. calculated a huge damage cost that mounted to US$ 10 trillion (50% of GNP) and estimated the increase in the number of death due to heat stress, tropical diseases and storms to be 3 millions. Such differences were caused by the methods these researchers chose and extrapolated information of low reliability. Although these incompatibilities are often seen and each of estimation has been criticized, many researchers agree on the seriousness of the future damage to human health.
As for the impact of global warming on human health, a number of studies have been conducted (such as WHO, 1990). These studies assert that there are comparatively direct influences on human health, such as an increase in heat stress, nutrition disorders caused by changes in food supply and changes in disease propagation mechanisms. Moreover, there are comparatively indirect
influences, such as stress increases by air and water pollution, disasters caused by sea level rise and the increase in floods. In relation to the increase in the death rate caused by heat stress Kalkstein (1989) estimated an increase of about 7000 deaths a year (only the urban areas of the U.S.) by climate changes based on the GCM calculation results by GISS. His study was based on an analysis of city mortality statistics in the U.S. and took into account adaptation to heat stress. Fankhauser extrapolated this result (a mortality rate of 4.5 in 100,000 people) to the whole world and estimated that the economic loss would amount to 31% of all the economic impacts by global warming. As for the impacts on food availability, Rosenzweig et al. (1994) conducted an international project, with the research organizations of 18 countries and concluded that the number of people at risk of hunger would be 640 millions in 2060 without global warming. And the global warming would increase this figure by 10%~60%. As for changes in infection mechanisms, various factors such as changes in vector ecosystems or deterioration of drinking water quality caused by hydrological changes (WHO, 1990) have been indicated. Malaria, schistosomiasis and dengue fever and other diseases shown in Table 2 are estimated to have great social impacts resulting from climate changes because their current impacts are serious and their climate sensitivities are high. Currently, the human population in malarial areas is estimated to be more than 2 billions, with the number of sufferers at 270 millions a year, and the number of deaths per year to be more than 1 million (WHO, 1992). It is still a disease with a grave effect on human health. Although the number of deaths it causes in the Sub-Sahara region is estimated to be quite high, information is unreliable and the exact situation is not clear. Other areas such as Afghanistan, Brazil, China, India, Mexico, the Philippines, Sri Lanka, Thailand and Vietnam account for 83% of the sufferers. However, some countries and areas have a growing death rate because of an increased number of refugees, public disorder and the appearance of chemically tolerant parasites. Also, it has been reported that malaria morbidity in these countries is connected with climate change caused by El Niño (Nicholls, 1993). It is believed that climate change resulting from global warming will worsen the situation. Figure 1 presents the latest malaria death rate.
Malaria is caused by malarial parasites when they enter the human body as sporozoite through the bite of the Anopheles mosquito. The malarial parasites are Protozoa. Important species causing malaria are Plasmodium falciparum, Plasmodium vivax, Plasmodium malariae, and Plasmodium ovale. The most dangerous one is Plasmodium falciparum. Its life cycle consists of pre-eryhrocytic phases in human liver cells and red blood cells plus sporogony. They are injected by mosquitoes and develop to microgametes and macrogametes in the mosquito's stomach. Microgametes fertilize macrogametes, to produce zygotes.
After fertilization, the zygote bores into the gutwall and forms a small cyst called an oocyst, in which sporozoites are produced. Eventually, the oocyst ruptures and liberates a great number of sporozoites. They invade the mosquito's salivary glands and are transmitted to humans when the mosquito bites again. Thus, propagation of the malaria parasite has two stages, one
each inside the bodies of both men and the Anopheles. It will not be directly affected by climate while inside a man. However, when inside the Anopheles, temperature has a great influence on its sporogony. Sporogony is impossible when the temperature is below 16°C and is impeded when it is over 32°C. The number of days needed for sporogony depend on the kind of parasite and on temperature and, for which MacDonald (1957) proposed the following formula:
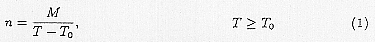
Here, n is the number of days needed for sporogony, T is temperature, M and T0 are constants decided by the kind of parasite, which, in the case of Plasmodium falciparum, are 111°C·d, 16.0°C. The ecology of Anopheles, the malaria vector, depends greatly on climate. About 400 species of Anopheles have been found so far, 10% of which are important malaria vectors. They belong to Holometabola and have 4 metamorphoses which are egg, larva, pupa and imago. The locations for egg lying vary with species; some prefer still water while others prefer running water. The area of activity sometimes extends to several kilometers away from breeding places. Availability of water is an important factor of their propagation. Adults are considered to be unable to survive the dry season. Their ecology changes greatly with temperature. Although they can survive a temperature of 12.7°C if the atmosphere is saturated, their optimum conditions is considered to be a relative humidity of 60% and temperature between 22°C ~ 30°C. Within this temperature range, their growth rate increases as the temperature rise. However, high temperatures shorten their life span and, so their numbers and population density declines (Dutta et al., 1978).
How are these relationships between climatic factors and life cycles of malaria parasites and Anopheles generally connected with malaria endemicity? To help analyze this, we will use the following symbols: m: the number of vectors per person, a: the frequency of bites of a vector per day, p: daily survival probability, r: the recovery and death rates of patients. The relationship between the average life length of vectors e and p is expressed by the following formula.
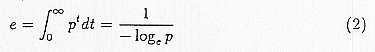
We will conduct an analysis of malaria infection using these symbols and the above formula. An infected person is bitten by m·a mosquitoes a day. The mosquito which is inoculated with gametocytes of malaria parasite needs to survive for more than n days, in order to obtain malaria endemicity. Thus, m·a·pn mosquitoes per day will have an endemicity from one person. Their average life span is 1/(-logep) and so they infect a person a day during that period. Accordingly, one infected person will produce C = ma2pn/(-logep) infected people each day. Considering such production and also allowing for recovery and death expressed with r, the differential equation for the number of infections P is as follows,
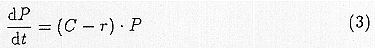
C is an index which Garrett-Jones et al. (1969) calls the vectorial capacity. In deriving this formula, there exist many assumptions. For example, the rate of infection is low and the following bite is of a non-infected person. The impacts of superinfection are not taken into account, the transfer of parasites by biting and their developments are assumed to be effective, the death rate of vectors is fixed independently of age, and the life-span of mosquitoes is not altered by infection. However, we consider C or C/r (= R0: the basic reproduction rate used by MacDonald) as a first approximates which represents the possibility of the occurrence of malaria. Thus, we assume that the impacts of climate change will effect malaria endemicity through parameters C or R0.
As mentioned above, m and n differ greatly with temperature, while m also depends on moisture. Although a and p have also been reported to vary with temperature and humidity, they have never been quantitatively evaluated. Thus, in this study, we assume that climate change will affect malaria endemicity through changes in m and n.
Figure 2 shows the assessment framework used in this study. The major components of the frame are the relationship between sporogony and temperature, and the ecoclimatic index model which shows climatic response of vectors. Complemented with these components, soil moisture submodule and outputs of equilibrium experiments of GCMs are attached.
The primary climatic variables of this framework are surface temperature and precipitation distributed spatially and temporally in both the current situation and that after climate change. The response of the ecoclimatic
index will be given as the change of m, and the response of sporogony to temperature will be given as the change of n. These are combined as the basic reproduction rate and used to estimate the changes in the areas of malaria occurrence.
Modeling the prevalence dynamics of malarious infection has been studied over a long time (Ross, 1977; MacDonald, 1957; Dietz et al., 1984). Research to establish the vector's population dynamics has also been conducted (Haile et al., 1977; Fine et al., 1979). However, these studies are aimed to support epidemic prevention and did not focus on climate change. Although some research on climate change (Haile, 1989; Sutherst, 1993) has also been conducted recently, mechanisms taken into consideration are limited in order to estimate the total figure of the impacts of malaria. In this study, we made an effort to integrated the knowledge and information of these former researches on the relationships with climate parameters, based on the framework given in Figure 2, and evaluated the impacts on global human health.
As showed in the previous section, the relative population density of Anopheles, as expressed by m, depends greatly on temperature and moisture. To describe the dependence quantitatively, it is necessary to estimate various parameters of their life cycle and to develop a population dynamics model based on them. However, with the knowledge available at this time, it is almost impossible to do so for each of the 40 species of vectors, and this situation will last for some time. Thus, we chose the simpler way than the above. We accepted the ecoclimatic index (EI) proposed by Sutherst et al. (1985) in order to estimate the impacts of climate change on m. EI is composed of climatic factors which are concerned with growth and with stress, and describes the favorability of climatic outcomes as their products.
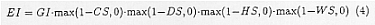
Where, GI is the index related to growth, CS is the stress caused by coldness, DS is that for dryness, HS is that for heat, and WS is the stress caused by humidity. GI is estimated by integrating the product of the temperature index TI and moisture index MI.
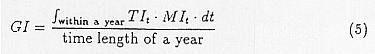
TIt is the product of IQ,t and IH,t,
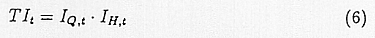
IQ is the normalized accumulated temperature which was calculated with the following formula for inter-diurnal variation of surface temperature.
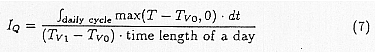
IH is the growth inhibition function which is linearly interpolated between the lower temperature Tv2 where inhibition starts and higher temperature Tv3 where the growth stops.
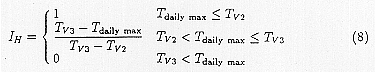
MIt is calculated from the soil moisture index SM ((soil moisture + surplus moisture)/field moisture capacity) using the following equation.
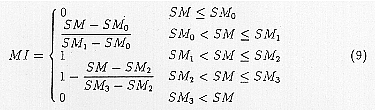
Where SM0, SM1, SM2 and SM3 are the parameters which describe the moisture characteristics of the mosquito. The stress indices are integrated values of the stress within a year which occur when climatic factors exceed their thresholds. The calculation was conducted each week as a unit time length. For example, CS is estimated as follows:
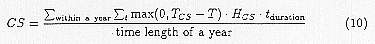
Here, TCS is the lower critical temperature, while HCS is its stress rate and tduration is the time after the stress begins. Stresses of dryness, heat and moisture are also expressed by a similar equation. Based on this equation the effects of stress increase rapidly when these conditions continue.
A lot of researches, such as the one by Dutta et al. 1978), on global ecology of Anopheles have been conducted. Using this research, we set each parameter that appears in EI as shown in Table 3. By the parameters, the suitable temperature for Anopheles is 29 ~ 30°C. They are exposed to severe temperature stress when the temperature is below 12.7°C and above 35°C, and cannot exist when it is over 40°C. The soil moisture parameters were based on the favorable conditions in its current distribution estimated using the soil moisture model which is explained in the next section.
For the analysis in the previous section, information on inter-annual and diurnal variation of temperature and soil moisture is necessary. In this study, we used the monthly mean temperatures calculated by Legates et al. (1989) for the current inter-annual temperature variation. As for the inter-diurnal variation, we calculated with diurnal temperature range compiled as databases by NCAR( ds512.0, 1979~1992, 7500 points) and CLIMEX by CSIRO (Maywald et al., l991), assuming a sine curved variation.
Soil moisture was estimated from a surface water balance model based on temperature, precipitation and field moisture capacity. This model estimates soil moisture W and evapotranspiration ET from monthly precipitation P, temperature T, and potential evapotranspiration PET. Soil moisture is dependent on the balance of precipitation Pr, snow melt Rs and PET. It can be increased to the value of the field moisture capacity FC in humid months when the sum of precipitation and snow melt are more than PET. For dry months, when this value is less than PET, we calculated it using a formula that assumes a linear relationship between [Delta] log SM and [Sigma] [PET- (Pr + Rs)] (Vorosmarty et al., 1989):
 using the following formula (Pastor <i>et al.</i>, 1984).<p>
<img src = )
As for the remaining surplus moisture that is in excess of the field moisture capacity, some is removed as surface runoff and the rest is stored as surface impoundment. Precipitation is treated as rainfall when the temperature is above 0°C and as snow when it is below 0°C. When the temperature is above 0°C the amount of snow melt is assumed to be 6 · T + 0.0125 · Pr · T (Sugawara, 1972). Potential evapotranspiration was estimated using the Thornthwaite equation. Although the use of this method on a global scale may be disputed, Mintz et al. (1993) and Vorosmarty et al. (1989) reported that it does not produce values that differ greatly from observed or estimated values by other methods. Field moisture capacity depends on vegetation and soil properties. On this, some research has been conducted and several sets of global data have also been published (Wilson et al., 1985, Webb et al., 1993, Bouwman et al., 1993). In this study, we compared the surface runoff estimated by the model used in this atudy with the above datasets and observed river discharges reported by McMahon (WMO Infoclima #1067), then adopted the most fitted moisture capacity datasets. The field moisture capacity we adopted was the lowest of the three values Webb et al. estimated from soil texture, soil-profiles, root-zone thickness. Figure 3 presents the distribution of the outflow volume estimated in our study and by MacMahon's. As for soil moisture and runoff, we picked up a typical one year result after several years' spin up calculation. Spatial resolution of 0.5° x 0.5° was used in water calculation and averaged to 1° x 1° grids when calculating the mosquito's model.
The climate parameters for global warming were obtained by adding the change profile from GCM calculations to the observed temperature, or multiplying ratio changes with the observed precipitation profile of Legates et al. (1989). As for diurnal ranges of temperature, we assumed no change. Table 4 presents the climate sensitivities of the 6 GCMs we used. Of these, GFDL R-30 was estimated at NOAA Geophysical Fluid Dynamics Laboratory, USA in 1989 and we used it as the standard case of this study, because it was the high resolution calculation of 3.75° x 2.23° and a number of calculation variables
were released. In the rest of this paper we exemplify the results of GFDL R-30 except where otherwise stated.
We stated in section 3 that the change in malaria endemicity could be estimated from the change in the basic reproduction rate R0. In this paper, we considered only the relative change in R0 and did not take its absolute values into account. Thus, we first normalized EI in an arbitrary range and assumed that its relative change is proportional to that of m. Also by assuming that the changes in r and a can be neglected, the change in R0 can be observed from the change in Rmodel, in the following equation. We focused on P. falciparam which has the highest death rate in four malarias. To make the calculation simple, we fixed p at 0.8(1/d).
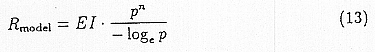
Figure 3 presents the Rmodel profiles for both the current climate and that when CO2 is doubled. We can see that the malarial area is not only concentrating in the three tropical areas of Africa, America and Asia, but also widely dispersed in China, Japan, the United States, Mediterranean nations and Australia. Values in the figure show 100 x Rmodel.
As for this calculation, what level is the significant for malaria epidemicity? China experienced extreme severe levels of malaria during the 1940's with about 30 million victims a year. During the period when eradication efforts were not enough, it was hyperendemic in areas south of 25N, and mesoendemic in areas between 25~33N and east of the Szechwan Basin (CACPD, 1989).
Figure 4 shows 100 x Rmodel and recent observed malaria morbidity (Zuou, 1981) in these areas, and we assume that the risk level of malaria becoming mesoendemic when Rmodel is over 1.0, and hyperendemic when Rmodel is over 6.5.
Figure 5 shows how the area where Rmodel is over 6.5 will expand because of global warming. Black areas are the current hyperendemic areas and gray areas are those in which malaria will become hyperendemic because of global warming. The fringelands of current malaria occurring areas such as the southern part of China, India, Tanzania and Brazil are absorbed in malarious area.
In Table 4, we summarize the populations of malarial areas estimated by this model. The current population in malarial areas (2.3 billions) is estimated to increase to 2.5 ~ 2.8 billions after global warming. In these estimations, we assumed the same population distribution as present.
Although malaria was eradicated in many areas in the 1950's and 60's, it was not possible to go on at the same pace after the 1970's, and now almost in stable equilibrium. However, malaria has occurred in some areas because of the migration of refugees due to lack of civil rest. As a result of the failure of eradication efforts, and the appearance of new distribution patterns of Anopheles caused by agricultural development and deforestation, the incidence
of malaria has increased in some areas. For example, although the number of victims in the Amazon at 1970 was 5100, it expanded to 1 million by 1990 due to deforestation and the influx of immigrants with no immunity. Thus, the present distribution and occurrence of malaria and the eradication efforts are hardly in balance. In this situation, as shown in Table 4, the potential areas of incidence will increase by 10 ~ 30% due to global warming of a doubled CO2 level. Furthermore, considering that this will happen in fringelands where the inhabitants have low immunity and that the disease will be brought by chemically tolerant parasites and vectors, the health of these people will be seriously affected.
The global burden of disease caused by malaria (shortened life spans due to disease and accidents after socio-economic adjustment) accounted for 2.6% of the total burden of all diseases in 1990 (World Bank, 1993). This ratio is greater than that of AIDS (1.4%) and traffic accidents (2.2%), and the increase caused by global warming accounts for a further 0.5%.
Measures to counter malaria have required a great number of people and expenses. Costs for studies, research and agricultural chemicals are estimated to be 0.2 ~ 0.4 US$ per person, while annual medical expenses per person
in countries with low incomes are estimated to be US$ 2 ~ 40. Although malaria infected areas have heavy expenses by the disease, this is not large in a global contest. Thus, we consider that it is possible to lighten or avoid those impacts on human health with appropriate policies and prudent foresighted planning.